Company
Trajectum Pharma is an innovative biopharmaceutical company, developing tolerance inducing mRNA therapies for autoimmune inflammatory diseases. The company is built on a deep-rooted expertise, built up over the past 4 decades at the Faculty of Veterinary Medicine, Department of Infectious Diseases & Immunology of Utrecht University.
Problem statement
Clinical manifestations of rheumatoid arthritis (RA) are manageable in 70% of patients, yet it is not curable, nor reversible. Current treatments are life-long and lead to high yearly costs (€25,000-30,000). The remaining group of patients suffers a range of side effects and secondary pathologies that make treatment a challenge, with due implications to the patient’s quality of life. Nearly all novel product development efforts are focused on improving disease management. Curative approaches are therefore highly desired.

The flow cytometry facility uses the Cytoflex LX from Beckman Coulter Life Sciences. With this machine single cells can be analyzed on an individual basis. For this purpose 6 lasers are available (blue, red, violet, yellow-green, near UV and infrared) with which 23 parameters can be studied per cell. This can be performed at a speed of 30.000 cells per second.
Solution
Trajectum Pharma is developing a potentially curative, first in class treatment, for which initial clinical proof-of-concept for the treatment of RA has been established.
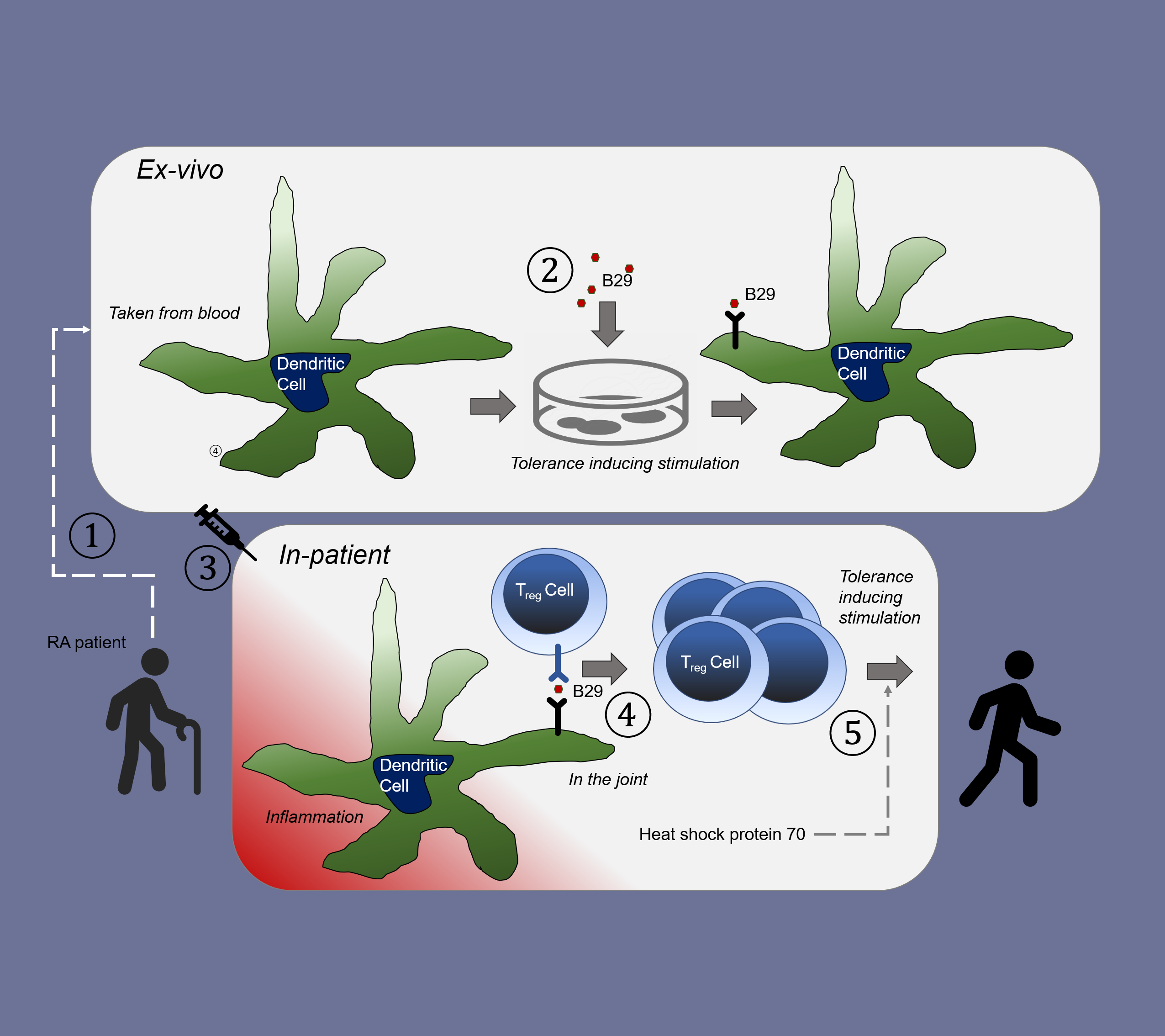
In RA patients, the approach consisted of ex vivo loading and modifying of dendritic cells (DCs) with Trajectum's proprietary peptide (B29, derived from a heat shock protein, HSP), and administration of the autologous DCs in RA patients. These loaded dendritic cells (tolDCB29) subsequently stimulate HSP-specific peripheral regulatory T-cells by releasing IL-10. This anti-inflammatory cytokine is typically present during inflammation at the site of inflammation (i.e. the joint of RA patients), restoring the balance.
We have demonstrated recently that the same effect can be brought about in an animal model, with mRNA, coding for the B29 peptide.
Path to succes
Trajectum Pharma has completed a clinical study (as yet unpublished), confirming the promising preclinical results and providing initial proof-of-principle in a small in nine RA patients. In parallel, we have obtained similar very promising results with an mRNA approach and we have an ongoing co-operation in the field of mRNA technology and lipid nanoparticle formulations with the Weissman Lab at UPenn (Philadephia, PA) (Prof. Drew Weissman)
The initial results in preclinical disease models are extremely promising. The long-term vision of the company is to develop an off-the-shelf vaccine for RA, using the principle of tolerance induction.
tolDCB29 therapy: Correcting repeated activation of disease-causing lymphocytes
Under natural circumstances, regulatory T-cell induction occurs to protect the body from overreacting to e.g. infection. In case of autoimmune diseases, these mechanisms are malfunctioning. Our approach is to mimic this natural self-controlling system by actively immunizing with the peptide B29 derived from heat shock protein (HSP), which has been shown to induce regulatory T-cells in vivo. Such T-cells are both prophylactically and therapeutically active. As physiological (natural) mechanisms of immunity are involved, the treatment concept can be considered as a (therapeutic) vaccine, and leads to the cure of the disease as opposed to transient suppression of symptoms.
Thus, the basic principle that underpins the therapy is to educate tolerance-invoking regulatory T-cells.
Meet Our Team

Paul Leufkens, PharmD, MBA, CEO
Paul holds MSc and PharmD degrees from Utrecht University (the Netherlands) and obtained his MBA at Bradford University (UK). Over the past 25 years he has spun out a variety of technologies from University-based research groups. Previously, he was a co-founder of AM-Pharma, Neurophyxia, CART-Tech, Space Medical Instruments, ICC HIFU Europe and Trajectum Pharma. He also held senior positions in product development at Exponential Biotherapies Inc. (Washington, DC), Reliance Life Sciences (Mumbai, India) and Pharming (Leiden, the Netherlands). Earlier in his career Paul worked in clinical development with Sanofi and Yamanouchi Europe. As a mentor to starting biotechnology entrepreneurs, Paul shares knowledge, experiences and pitfalls from his area of expertise (biotechnology, medical devices and clinical development).and he is a jury member and due diligence expert for the European EIC Accelerator grant.program.

Prof. Dr. Willem van Eden, MD, Scientific Director
Willem is immunologist and medical microbiologist and has made a set of landmark discoveries at the edge of infectious diseases and autoimmunity. He discovered the role of stress proteins in self-tolerance. This serendipitous finding was published in Nature and was for two years in the top-100 of mostly cited paper in the biomedical domain. Resulting from this original finding are still current. Activities of van Eden and his group directed towards development of an immuno-modulatory vaccine against chronic inflammatory diseases on the basis of heat shock proteins.

Prof. Dr. Jaap van Laar, MD, PhD, Principal Investigator, Tolerant Study
Jaap is Head Rheumatology and Clinical Immunology Department at the UMCU and he is involved with Trajectum Pharma as the Principal Investigator of the Tolerant Phase I/IIa clinical study Dr. van Laar has a vested interest in the use of heat shock proteins in immune-mediated therapies, as his career started in this field. Throughout his career, he has been closely involved in many projects that bridge development and practice. He has been the lead investigator in numerous clinical trials in the area of rheumatology.

Prof. Dr. Femke Broere PhD, DMV, Head of the department of Infectious Diseases and Immunology, Faculty of Veterinary Medicine, Utrecht University
Femke is heading the preclinical development effort at her department in support of the preclinical and clinical studies, including the areas of assay development and data-management and evaluation of study results.
.

